Abstract
Congenital Dyserythropoietic Anemias (CDAs) are a heterogeneous group of rare hereditary anemias caused by ineffective and dyspoietic erythropoiesis, with the pathognomonic feature of binuclear and/or multinuclear erythroid precursors in the bone marrow. The clinical diagnosis of CDA is based on identifying the characteristic morphology of erythroblasts in the bone marrow of patients presenting with chronic anemia, frequently with evidence of hemolysis but suboptimal reticulocytosis, and iron overload. CDAR (ClinicalTrials.gov identifier: NCT02964494), a registry for patients with CDA in North America, has been established with the goal of providing a longitudinal database and associated biorepository to facilitate natural history studies and research on the pathogenetic mechanisms in this heterogenous disease.
A 2-year-old female, enrolled in CDAR, had transfusion-dependent anemia since birth. She demonstrated accelerated iron overload and had failure to thrive despite regular transfusions maintaining trough hemoglobin higher than 9.5 g/dL. Bone marrow evaluation revealed dyserythropoiesis along with ring sideroblasts (RS), resembling acquired myelodysplastic syndrome (MDS) with the characteristics of Refractory Anemia with RS (RARS). Whole-exome sequencing for the proband and her parents in trio analysis identified a de novo, heterozygous germline DHX38 missense variant c.3310A>C, p.Thr1104Pro in the proband. In silico prediction programs suggest this rare variant is deleterious.
DEAH box RNA helicase DHX38 is an RNA helicase, homolog to the yeast Prp16 that has been shown to play a dual role in the spliceosome complex: an ATP-independent role in the first catalytic step mediating proofreading of the branchpoint sequence, and an ATP-dependent role in the second step, mediating removal of the lariat and joining the exons to complete the splicing (Tseng et al, RNA 2011). DHX38 is highly expressed during terminal erythropoiesis similarly to SF3B1, the splicing factor gene most frequently mutated in MDS-RARS.
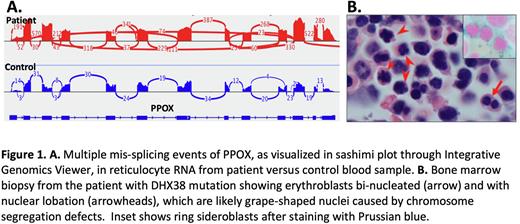
RNA-seq analysis of CD71+ reticulocytes isolated from the proband at three different time points compared to three different normal control samples, using AltAnalyze software, revealed approximately 3,000 differentially expressed genes. More than 1,500 differentially spliced events were identified, affecting over 200 genes, including SLC25A37, coding for an iron importer localized in the mitochondrial inner membrane and PPOX, coding for protoporphyrinogen oxidase (Figure 1A), both essential for the synthesis of mitochondrial heme. Mis-splicing of the same genes is a characteristic finding in MDS-RARS associated with SF3B1 pathogenic variants, most commonly p.Lys700Glu, and may contribute to RS formation.
Moreover, DHX38 is known to interact with centromeric noncoding RNA, regulating chromosome segregation during mitosis, and its depletion in vitro causes abnormal grape-shaped nuclear morphology (Nishimura et al, Genes Cells 2019). Review of the patient's bone marrow studies revealed several erythroblasts with grape-shaped nuclei (Figure 1B). Erythropoiesis cultures from patient-derived iPSCs were utilized to phenocopy the disease in vitro. Cytospins of the generated erythroblasts revealed dyserythropoiesis that was verified quantitatively by increased frequency of nuclear abnormalities in erythroblasts produced in vitro from patient-derived iPSCs analyzed by imaging flow cytometry. Extra-hematopoietic abnormalities of the nucleus and mitotic defects were explored in fibroblasts generated from the patient's skin biopsy, which also tested positive for DHX38 p.Thr1104Pro. Grape-shaped nuclei were detected in cultures of patient's fibroblasts indicating improper mitotic exit due to aberrant chromosome segregation.
Our findings indicate that DHX38 is likely the causative gene for this CDA with a RARS phenotype, causing mis-splicing of a multitude of genes critical for iron metabolism and erythropoiesis as well as defects in chromosome segregation and cell division. Out of approximately 250,000 alleles in the gnomAD database, the DHX38 p.Thr1104Pro variant has been detected in 4 alleles; however, SF3B1 p.Lys700Glu is also detected in 22. Therefore, the rare presence of this DHX38 variant in "healthy" population may indicate that acquired DHX38 pathogenic variants exist and could be responsible for SF3B1-negative MDS-RARS.
Disclosures
Lutzko:Elixirgen Therapeutics: Other: Industry sponsored Clinical trial research . Kalfa:Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Forma Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal